13 Epic How To Find The Density Of A Gas - After you have found the volume, you must find the mass. The specific volume v is given by:
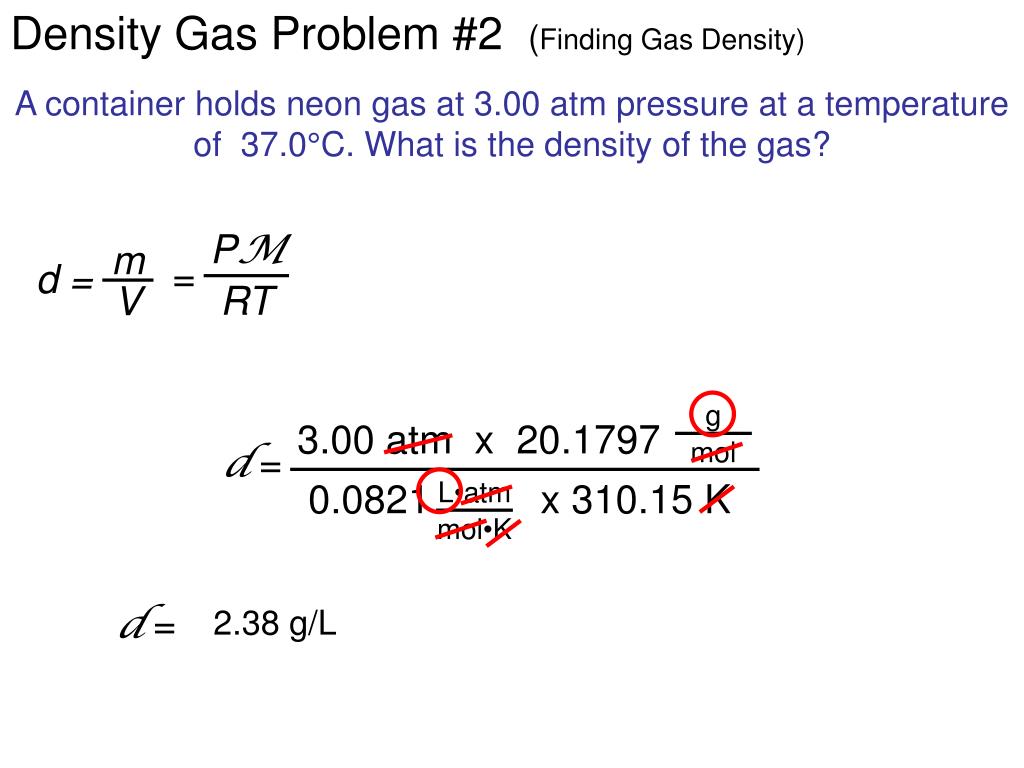 How To Find Density Of A Gas Given Temperature And Pressure . Hydrogen, molecule h₂, molecular mass 2, vapour density 2/2 = 1 oxygen, molecule o₂
How To Find Density Of A Gas Given Temperature And Pressure . Hydrogen, molecule h₂, molecular mass 2, vapour density 2/2 = 1 oxygen, molecule o₂
How to find the density of a gas

7 Results How To Find The Density Of A Gas. To find out the 2d density of states, according to the definition 1, we must first find out the number of available states in the 2d electron gas. But for gases, the density can vary over a wide range because the molecules are free to move. Universal gas constant [r] the density of gas is defined as mass per unit volume of a gas under specific conditions of temperature and pressure. How to find the density of a gas
Here is the ideal gas law equation. To find the density of the gas, you need to know the mass of the gas and the volume. Estimate the density (in $\pu{kg/m^3}$) of the gas. How to find the density of a gas
Here is a general strategy for getting a quick and dirty estimate of gas densities: If we know the density, total pressure and temperature of the gas the molecular mass can be easily determined from this equation. Finding the density of a gas is the same as finding the density of a solid or liquid. How to find the density of a gas
He discovered that a body immersed in a fluid, experiences an upward push equal to the weight of the fluid it displaced. Moreover, how do you find the density? A syringe with a mass of 45.861 g is filled with 50.0 ml of carbon dioxide at the lab temperature and pressure (1.00 atm and 20 °c). How to find the density of a gas
Density is mass over volume. At stp, one mole of Because the density of the gas is less than 1 g/l, the molar mass is less than 22.4 g/mol. How to find the density of a gas
Density is the measurement of the amount of mass per unit of volume. An important property of any gas is its density.density is defined as the mass of an object divided by its volume, and most of our experiences with density involve solids. In addition, the density of a gas is equal to its mass divided by its volume. How to find the density of a gas
For example, you could fill a syringe of known mass and volume with the gas and measure the mass of the gas. The answer is the density compared with hydrogen gas. One way to measure the density of a gas is to take a given volume of the gas and determine its mass. How to find the density of a gas
In most cases, however, the exact number of states is not known. The density is determined by utilizing a variation of the ideal gas law where density and molar mass replace moles and volume. 3 we provide the reciprocal area occupied by these states. How to find the density of a gas
The tricky part with gases is that you are often. Where m a is the molecular mass of the gas and d is the density of the gas. The gas consists of (by volume) $20\ \%$ of $\ce{ch4}$ and $80\ \%$ $\ce{n2}$. How to find the density of a gas
Universal gas constant is a physical constant that appears in an equation defining the behavior of a gas under theoretically ideal conditions. To find the density of the gas, you need to know the mass of the gas and the volume. Ideal gas law formula the traditional method of computing the molar mass of any substance, regardless of its phase, is to find the sum of all atoms that constitute the substance. How to find the density of a gas
We can determine it, however, if in addition to eq. 27 degrees celsius + 273 = 300 kelvin. You have to know the mass and the volume of the gas. How to find the density of a gas
Work out the molecular weight of the substance and divide that by 2. 12 votes) molar mass is equal to density (in g/l) multiplied by molar volume. After you have found the volume, you must find of. How to find the density of a gas
The original ideal gas law uses the formula pv = nrt, the density version of the ideal gas law is pm = drt, where p is pressure measured in atmospheres (atm), t is temperature measured in kelvin (k), r is the ideal gas law constant. Density is mass per unit volume. The density of the gas is equal to its mass divided by the volume. How to find the density of a gas
Here is the ideal gas law equation rearranged to solve for v: For example, if you were looking for the density of water vapor, you would divide 18 g/mol by 22.4 l/mol to yield 0.804 g To find a given gas density, divide the molar mass of the gas by the molar volume (22.4 l / mol in this case). How to find the density of a gas
You can take advantage of the observation made by our old friend archimedes. Complete answer to this is here. Here is the ideal gas law equation rearranged to solve for v: How to find the density of a gas
I can't just use the ideal gas law to find out the density of the mixture as there are two from the To solve the problem, you have to find the mass of a sample of unit volume. To find density, we have to solve the equation for volume, or v.v = nrt / p.to incorporate. How to find the density of a gas
Density of gas given pressure and temperature of gas. How to find the density of a gas
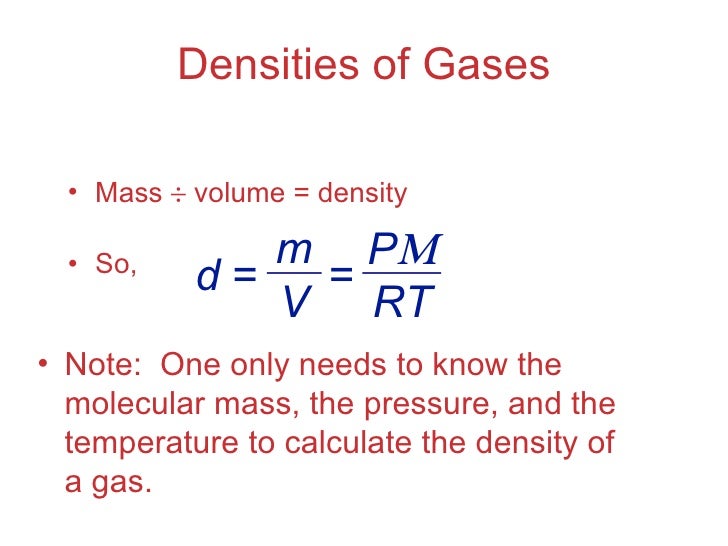 Chapter 10 Lecture Gases . Density of gas given pressure and temperature of gas.
Chapter 10 Lecture Gases . Density of gas given pressure and temperature of gas.
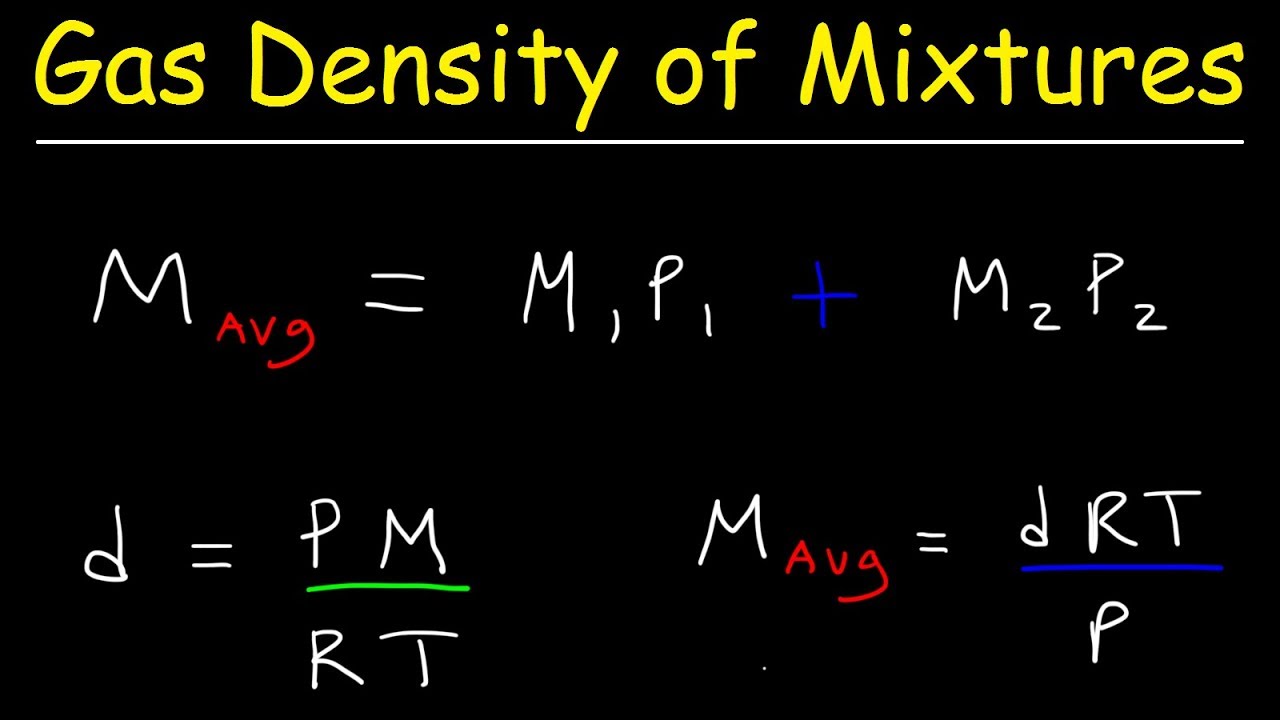 How To Find Density Of A Gas Given Temperature And Pressure . To find density, we have to solve the equation for volume, or v.v = nrt / p.to incorporate.
How To Find Density Of A Gas Given Temperature And Pressure . To find density, we have to solve the equation for volume, or v.v = nrt / p.to incorporate.
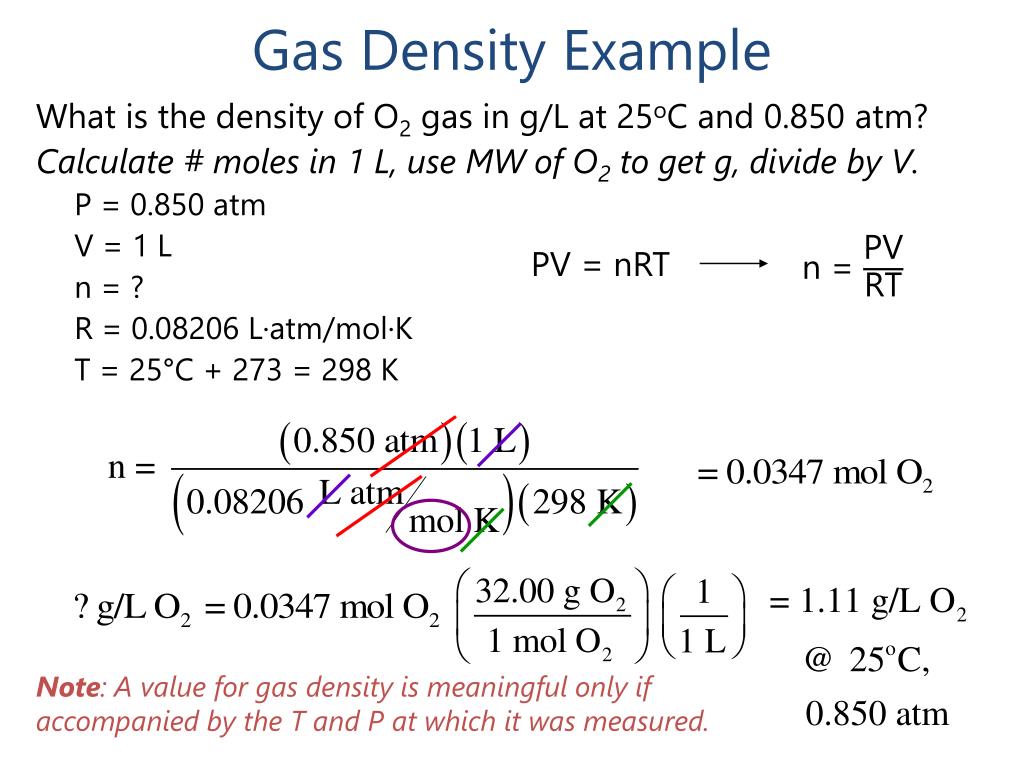 PPT Standard T & P (STP) PowerPoint Presentation, free . To solve the problem, you have to find the mass of a sample of unit volume.
PPT Standard T & P (STP) PowerPoint Presentation, free . To solve the problem, you have to find the mass of a sample of unit volume.
 Standard Temperature and Pressure Chemistry Video . I can't just use the ideal gas law to find out the density of the mixture as there are two from the
Standard Temperature and Pressure Chemistry Video . I can't just use the ideal gas law to find out the density of the mixture as there are two from the
 How To Find Density Of A Gas In Chemistry . Here is the ideal gas law equation rearranged to solve for v:
How To Find Density Of A Gas In Chemistry . Here is the ideal gas law equation rearranged to solve for v:
 How To Find Density Of A Gas At Stp . Complete answer to this is here.
How To Find Density Of A Gas At Stp . Complete answer to this is here.

Comments
Post a Comment